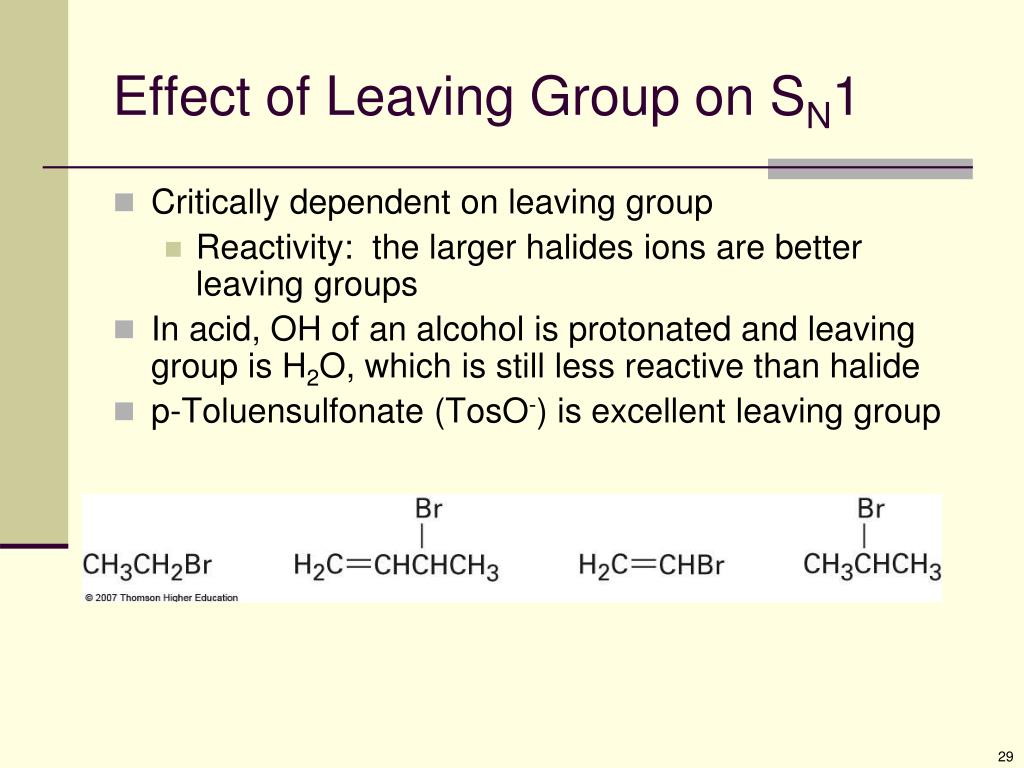Pankaj singh chemistry expert explains why aryl halides are less reactive than alkyl halides for nucleophilic substitution.
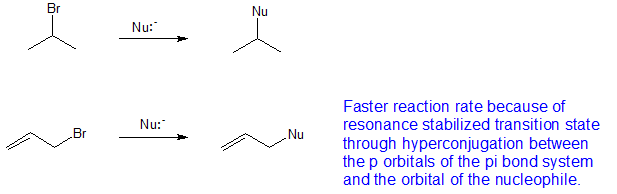
Why allyl halides are more reactive than vinyl halides.
The sp2 hybridised carbon atom with greater s character is more electronegative.
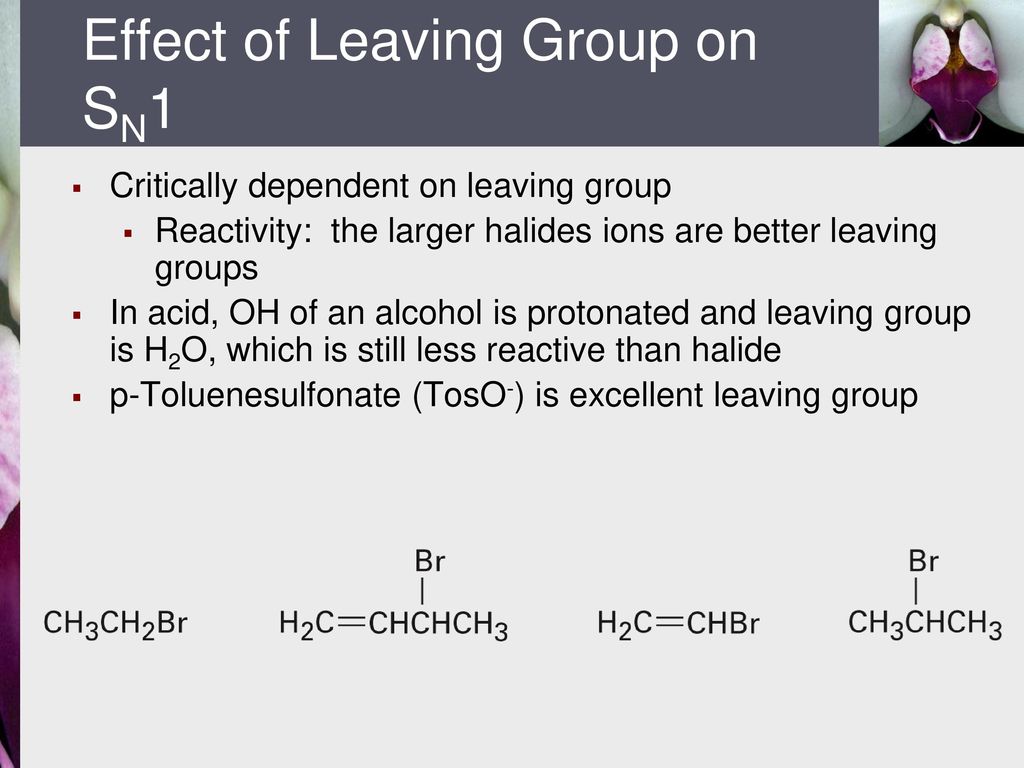
Vinyl halides are less reactive than alkyl halides.
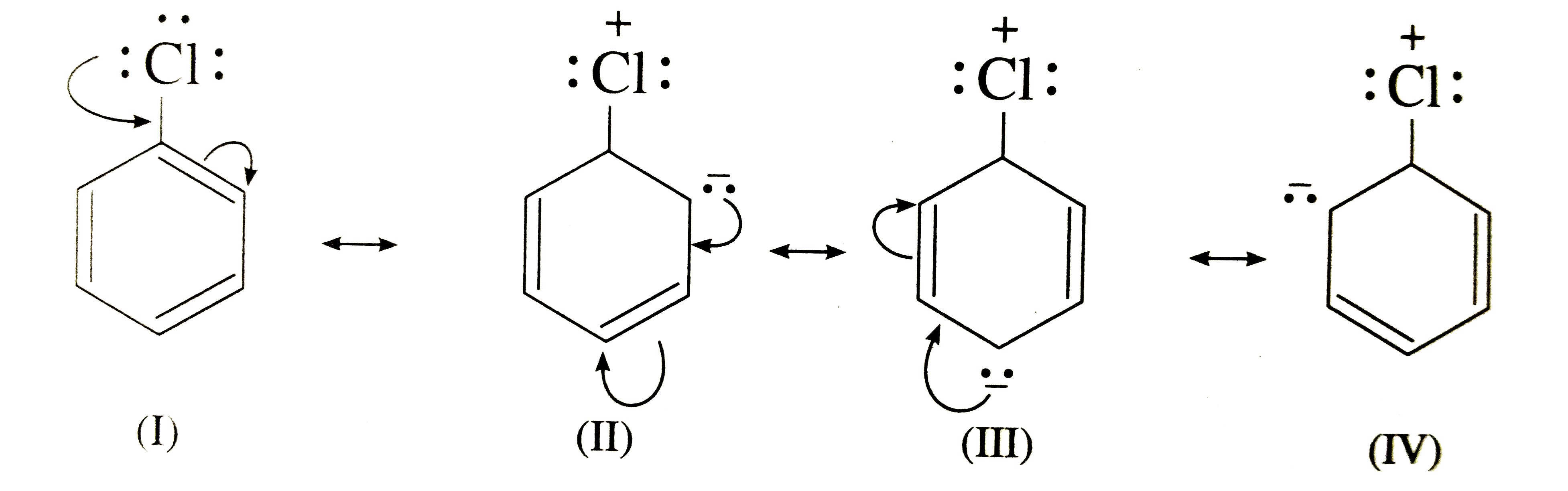
Following factors are responsible for the low reactivity of aryl halides towards nucl.
This is because c x bond in vinyl halides have partial double bond character due to resonance.
So it is difficult to break the c x bond.
Halo alkane and haloarene organic chemistry class 12 compounds.
In allyl halides the double bond is in conjugation with lone pair on halides.
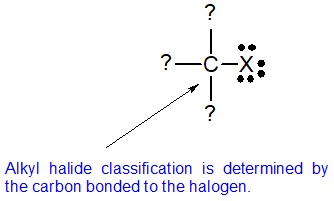
In alkyl halides carbon atom of c x bond is sp3 hybridised while in aryl halides the carbon atom attached to halogen is sp2 hybridised.
The thyroid hormones t 3 and t 4 are exceptions.
Whereas alkyl halides undergo nucleophilic substitution easily.
As is fluoroacetate the toxic agent in the south african shrub dichapetalum cymosum known as gifblaar however the halogen rich environment of the ocean has produced many interesting natural products incorporating large.
It can hold the electron pair of the bond more tightly than sp3 hybridised carbon atom in alkyl halides with less s character.
Aryl halides are less reactive towards nucleophilic substitution reaction due to several reasons.